A computational study of room-temperature ionic liquids interacting with phospholipid bilayers
Richard Bingham and Pietro Ballone, Queen's University Belfast
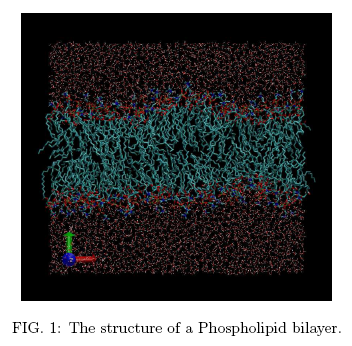
Phospholipid bilayers (Fig. 1), stabilised by their interaction with an aqueous medium, represent the major structural component of bio-membranes surrounding cells and subcellular organelles. The role of this molecularly thin, flexible and yet resilient envelope goes beyond containment and protection, since it also regulates the flow of nutrients and waste compounds in and out of cells, and contributes to the exchange of chemical and sometimes electrical signals among cells. Last but not least, bio-membranes provide the physical support for a variety of energy conversion processes powering life on Earth.
The structural and functional integrity of this essential cellular component is imperative, and hence a primary factor determining the biological effect of any new chemical species synthesised, is precisely its interaction with bio-membranes.
In this respect, a case in point is provided by the so-called room-temperature ionic liquids (RTILs) - a vast class of organic ionic materials, currently investigated because of their high potential for applications. Widespread usage of these compounds in industrial processes would certainly result in their intentional or unintentional release into the environment.
Even though RTILs are heralded as the spearhead of a new and greener chemistry, it is nevertheless necessary to assess the biological effect of each of them. Concerns about their toxicity have been highlighted by experimental studies that revealed strong association of RTILs with bio-membranes, leading at high concentration to the disruption of the phospholipid bilayer, especially in the case of RTILs with long hydrocarbon side chains. It should be noted that the RTIL concentrations considered in these measurements are orders of magnitude higher than those expected to arise in biological tissue from environmental pollution. Still, the experimental observations do point to aspects that need a closer scrutiny, especially taking into account that the complexity of living organisms, together with the intricate interdependence of biochemical pathways, almost guarantee that unexpected effects are the most likely result of introducing new chemicals into our environment. Alternatively, strong and specific interaction with membranes of ionic and, in some cases, amphiphilic compounds such as RTILs might have fundamental implications for pharmacology applications or biomedical technologies concerning, in particular, novel strategies to deliver drugs to cells across their protective membrane.
The state of the art in molecular modelling and high performance computing is such that computer simulation can play a relevant role in assessing the interaction of RTILs with lipid bilayers, and might provide a working tool for a first preliminary screening of RTILs on the basis of their expected interaction with bio-membranes. Additionally, simulation can advance our understanding of the microscopic mechanisms underlying positive or negative effects of RTILs on the stability of bio-membranes.
ACKNOWLEDGEMENTS
Computations have been made possible by a Class 1b EPSRC-HPC grant. All simulations have been carried out using Gromacs 4.5.5/4.5.3, available on Hector. R. B. is supported by a Leverhulme Trust Research Project Grant (F/00 203/Y)