A quantum theory of hydrogen bonds
Xinzheng Li, Brent Walker, and Angelos Michaelides, London Centre for Nanotechnology & Department of Chemistry
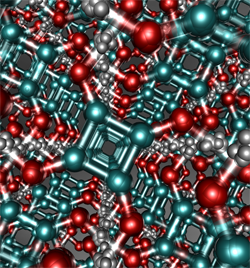
Figure: Squaric acid, a strong hydrogen bonded crystal, which possesses
delocalised hydrogens (small grey spheres) in a strong covalently bonded framework
of carbon (turquoise spheres) and oxygen atoms (red spheres). Quantum effects
cause the hydrogen bonds holding this material together to strengthen.
Hydrogen (H) bonds are essential to life on earth. They are, for example, the main intermolecular interactions responsible for binding the two strands of DNA and holding together the condensed phases of water. Because H is the lightest element, it exhibits unusual quantum properties such as the ability to “tunnel” through energy barriers. These quantum properties make H-bonds highly sensitive to isotope changes. Isotopes are atoms that contain the same number of protons but a different number of neutrons. Deuterium is the most common isotope of H and when H-bonds involve deuterium rather than H something strange happens: some get longer, some get shorter and some show no change at all. This behaviour has remained an enigma since its first detection in the 1950s.
In their paper Li et al. solve this puzzle by showing that the influence isotope effects have - and in essence quantum effects - on H-bonds depends on their strength: strong H-bonds get stronger and weak H-bonds get weaker. This surprisingly simple finding is arrived at on the basis of state-of-the-art computer simulations on the UK’s largest supercomputer (HECToR) in which both the electrons and the nuclei are treated as quantum mechanical objects. It rationalises in a clear manner the seemingly conflicting results previously reported on different classes of H-bonded systems (gas phase clusters, solids including ferroelectrics, and liquids).
Detailed analysis of the underlying physics shows that the correlation arises from competition between covalent (intramolecular) bond stretching and intermolecular bond bending – different modes dominate in strongly and weakly bonded systems.
The simple rule of thumb identified in Li et al.’s work could in the future be used by experimentalists to interpret measurements on isotope effects in H-bonded liquids, ferroelectric phase transition temperatures in H-bonded crystals, high pressure phases of ice, and proton transfer probabilities in H-bonded biological materials (e.g. beta sheets and alpha-helixes).
Further information, acknowledgements and links
- For more information see Li et al. Proc. Nat. Acad. Sci. 108, 6369 (2011) or
www.chem.ucl.ac.uk/ice.
Also a movie summarising the main findings of the paper can be seen at Quantum hydrogen bonds - Some other work of the research group performed on HECToR is summarised in these movies:
Surface mediated proton transfer
Fully Quantum Molecular Dynamics Simulations of Molecules on a Metal Surface: Part I
Fully Quantum Molecular Dynamics Simulations of Molecules on a Metal Surface: Part II