New tricks from old oil: what can biofuels learn from the catalysis of crude oil formation?
Dr Dawn L. Geatches, Prof. Stewart J. Clark, Dr. H. Christopher Greenwell, Durham University, South Road, Durham
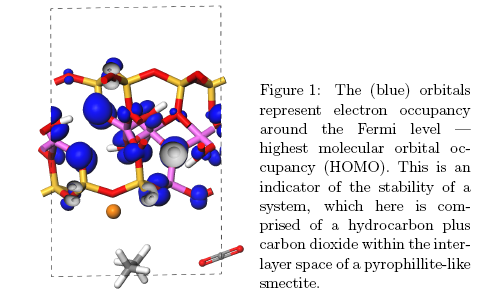
Quantum mechanical investigations can probe molecular environments in a far more controlled way than is possible experimentally, for example, investigations at higher pressures than are possible within a laboratory are straightforward. Computations can provide information where experimental analyses encounter difficulties, such as determining the position of hydrogen atoms. In our study into fossil oil and biofuels, we used the first principles quantum mechanics code, CASTEP (1) to investigate a decarboxylation reaction.
CASTEP employs planewaves to describe the quantum mechanical behaviour of electrons where the usual requirement is several thousand planewaves within any one calculation. Atomistic simulations using planewaves are processor-intensive, scaling approximately with the cube of the number of atoms in a system. CASTEP has been designed as a parallel code, which optimises processing effciency consequently enabling quantum mechanical computations that were once impossible. To investigate the conversion of a fatty acid into a hydrocarbon i.e. decarboxy- lation, we created the clay mineral models using Materials Studio An example of the models is shown in Figure 2, which is a schematic of the intermediate stages in the decarboxylation of a model fatty acid, as theorized by Almon and Johns [2]. Each model was structurally relaxed from these initial configurations, and four clay mineral environments were examined.[3]
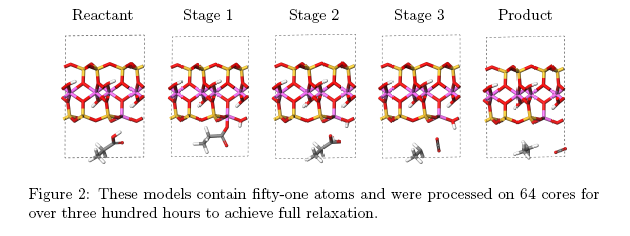
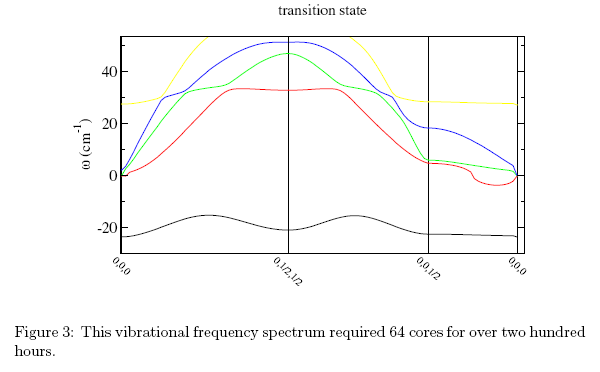
Figure 3 shows an example of how the frequency of vibration of a material - in this case a transition state in the decarboxylation pathway - varies with sampling points throughout the model. Identifying a transition state in a chemical pathway provides information about the experimental conditions that produced it, thereby offering potentially greater control over the experimental process.
These results form the basis of future research into the creation of a more complex chemical environment - and hence more processor-intensive - for the investigation of decarboxylation, with the ultimate goal of informing the production of biofuels.
References
- [1] S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson, and M. C. Payne. Castep v5.0. Zeitschrift fur Kristallographie, 220(5-):567 - 570, 2005.
- [2] W. R. Almon and W. D. Johns. Petroleum forming reactions: the mechanism and rate of clay catalyzed fatty acid decarboxilation. In 7th International meeting on organic geochemistry, volume 7th, pages 157 - 171, Chichester, September 1975. Wiley.
- [3] D. L. Geatches, S. J. Clark, and H. C. Greenwell. Role of clay minerals in oil- forming reactions. J. Phys. Chem. A, 114:3569 - 3575, 2010.